Sythonics and MCP Science
Science
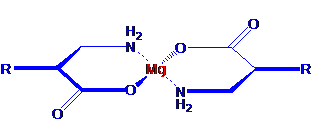
The first figure shows an MCP created by chelating, or binding at multiple sites, a single magnesium atom to two molecules of an active pharmaceutical agent (API).
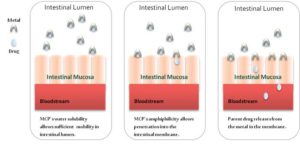
In its simplest application, metal coordination improves a drug’s absorption and delivery without changing the way the drug works once delivered. Orally ingested drugs must typically pass through both a water-based environment (the intestinal tract) and an oil-based environment (the intestinal lining) in route to their intended targets. Most pharmaceutical compounds cannot pass efficiently through both of these environments, and traditional efforts to improve a compound’s solubility in one environment typically diminish its solubility in the other. For example, creating a salt of a compound typically increases its water solubility but decreases its ability to pass through oils. Metal coordination, however, can improve solubility in water while maintaining the solubility in oils necessary to permit efficient passage from the digestive tract to the bloodstream and the target cell. The figure below shows the metal ion acting as a “chaperone” that helps the drug enter the bloodstream more quickly and efficiently than it otherwise would.
Performance Enhancements
Manipulation of a drug’s pharmacokinetics through metal coordination can provide clinically significant benefits. With MCP we have demonstrated the following performance enhancements:
- Accelerate the onset of action of an OTC product.
- Reduce the variability of absorption of the relatively insoluble drug, furosemide.
- Impart bioadhesive properties to extend levodopa’s release.
- Combine multiple agents to a single molecular entity to advance our Frequency Modulated Drug Delivery platform technology (FMDD).
- Improve efficacy of IV delivered drugs.
- Localize delivery to and prevent the systemic absorption from the GI tract of drugs to treat ulcerative colitis.
Capabilities
Metal coordinated pharmaceuticals (MCPs) can be used to overcome specific clinical deficiencies of currently marketed products as well as those in development. Our lead drug candidate, MCP-311, provides a new solution to extending the absorption phase of levodopa using the bioadhesive properties of a bismuth-levodopa co-polymer. This allows a reduction in pulsatility of levodopa plasma levels to achieve continuous dopaminergic stimulation for Parkinson’s disease patients. Alterations of physicochemical properties that accompany the coordination of a metal to a drug can improve the drug’s amphiphilicity (achieving improved solubility in both aqueous and lipid environments) and which can be applied to achieve a faster onset of action, minimize absorption variability and improve drug distribution from blood to target organs such as lungs. One drug Synthonics worked with could only be given intravenously in a solution with an uncomfortably high pH. Our chemistry was able to improve water solubility to allow dissolution at more neutral pH ranges. Thus, an orally administered drug could now be given parenterally. In other applications, metal coordination can enable two drugs to be delivered simultaneously to exploit synergistic activity and to prevent systemic absorption when the drug needs to remain in the lumen of the GI tract. As we expand our research, we continue to find new applications for MCPs to solve problems related to pharmacokinetic properties that lead to other clinical deficiencies.
Key theory/thinkers that paved the way for Synthonics
COORDINATION COMPLEX
In chemistry, a coordination complex consists of a central atom or ion, which is usually metallic and is called the coordination centre, and a surrounding array of bound molecules or ions, that are in turn known as ligands or complexing agents. Many metal-containing compounds, especially those of transition metals, are coordination complexes. A coordination complex whose centre is a metal atom is called a metal complex.
ALFRED WERNER
Alfred Werner was a Swiss chemist who was a student at ETH Zurich and a professor at the University of Zurich. He won the Nobel Prize in Chemistry in 1913 for proposing the octahedral configuration of transition metal complexes. Werner developed the basis for modern coordination chemistry. He was the first inorganic chemist to win the Nobel prize, and the only one prior to 1973.
NEPHELAUXETIC EFFECT
The nephelauxetic effect is a term used in the inorganic chemistry of transition metals. It refers to a decrease in the Racah interelectronic repulsion parameter, given the symbol B, that occurs when a transition-metal free ion forms a complex with ligands. The name ‘nephelauxetic’ comes from the Greek for cloud-expanding. The presence of this effect brings out the disadvantages of crystal field theory, as this accounts for somewhat covalent character in the metal-ligand interaction.
ORGANOMETALLIC CHEMISTRY
Organometallic chemistry is the study of chemical compounds containing at least one chemical bond between a carbon atom of an organic compound and a metal, including alkaline, alkaline earth, transition metal, and other cases. Moreover, some related compounds such as transition metal hydrides and metal phosphine complexes are often included in discussions of organometallic compounds. The field of organometallic chemistry combines aspects of traditional inorganic and organic chemistry.
COORDINATION GEOMETRY
The term coordination geometry is used in a number of related fields of chemistry and solid state chemistry/physics.
